Starting with
Master of Science thesis at the Warsaw University through Ph.D.
studies in the Institute of Physics Polish Academy of Sciences and
first
postdoctoral appointment at the University of Cincinnati I have been
involved in studying the optical properties of various semiconductor structures,
mainly quantum wells and quantum dots. However, somewhere in the process,
seeing the importance of interdisciplinary research, I decided to gain
experience in another, rather different discipline. As a result of this
sudden twist, I am currently an Alexander von Humboldt research fellow
in the Biophysical Chemistry Group at the Department of Chemistry and Biochemistry,
Ludwig-Maximilian University Munich.
Briefly, the
focus of my research is to study single pigment-chlorophyll-protein complexes
(in particular peridinin-chlorophyll-a-protein, PCP) using single molecule
spectroscopy. More extended description of the first results could be found
here.
1. Introduction.
Proteins are
biological molecules composed of linear chains of amino acids. This chain
structure, even for small proteins, results in a large number of spatial
degrees of freedom and possible structural configurations (conformations).
Since each configuration of the amino acids corresponds to specific energy
of a protein, the resulting energy diagram of the protein features a complicated
and multidimensional landscape.
In order to
succinctly describe dynamics of the protein folding, a hierarchical energy
funnel model has been proposed [1]. In this model, the structure of a protein
fluctuates within the family of conformations characterized by similar
energy while the transfer between different families occurs via non-equilibrium
thermally activated transitions [1]. Protein folding is then a continuous
transition from one conformation state to another, presumably with progressively
lower energy. The photodissociation experiments, where large ensembles
of myoglobin have been investigated, have indeed supported the energy funnel
model [2,3]. However, a detailed description of the protein energy landscape
requires the removal of the inhomogeneous broadening always present in
ensemble experiments [4].
The primary
objective of my project is to apply single molecule spectroscopy to probe
structural changes of chromophore - protein complex via monitoring the
optical transitions of a single chromophore. Since such complexes are usually
quite unstable and feature relatively low fluorescence quantum yield, I
intend to improve collection efficiency of single molecules by introducing
solid immersion lens (SIL).
2. Low Temperature Single Molecule
Spectroscopy.
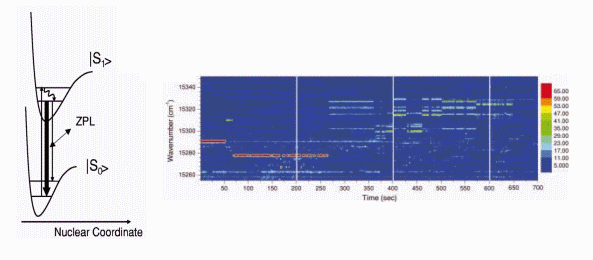
In the pioneering
papers it has been shown that low temperature single molecule spectroscopy
is a powerful tool for probing interactions between an individual chromophore
and its local environment [5]. Indeed, the influence of the surrounding
has been inferred through monitoring the behavior of the zero-phonon-line
(ZPL) absorption energy of a single molecule, while scanning the
wavelength of the narrow-band excitation laser. Any modification of the
chromophore’s environment can be observed through changes in either the
energy or the intensity of the ZPL absorption. However, an important drawback
of this method is that only molecules characterized with a sharp and spectrally
stable ZPL absorption can be studied, which excludes most fluorescing systems,
including fluorescent proteins.

3. Peridinin – Chlorophyll-a
– Protein Complex.
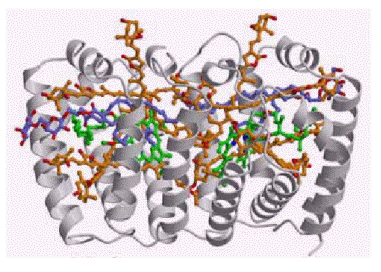
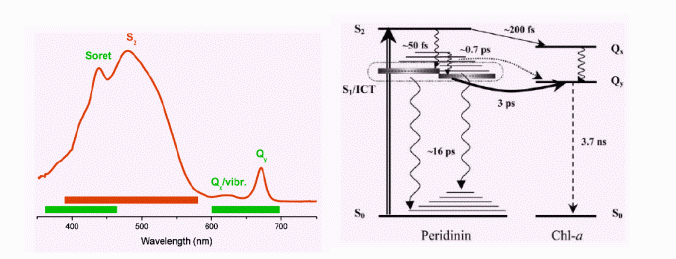
Peridinin-chlorophyll-a-protein
(PCP) complex is a photosynthetic molecule present in dinoflagellate Amphidinium
carterae [7]. It is composed of a barrel of hydrophobic protein, which
shields two chlorophyll-a molecules, each dressed by four peridinins (Fig.
2).
4. Solid Immersion Lens.
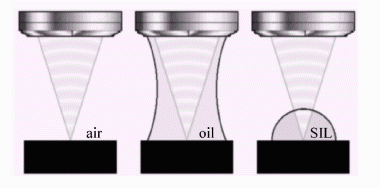
One of the limitations
of single molecule spectroscopy at cryogenic temperatures concerns usually
low numerical aperture (NA) of a microscope objective. Low NA, while decreasing
collection efficiency leads also to a large excitation volume and results
in a more intense background. I will use a hemispherical solid immersion
lens (Fig. 4), which will be placed between the microscope objective and
the sample [9,10]. In this way, similarly to the oil immersion microscopy,
the NA can be increased by n, where n is the refractive index of the SIL
(in my case, 1.83). Since the working distance of the objective is only
0.4 mm, the SIL of a diameter of 0.5 mm will be used.
References
[1] H. Frauenfelder, S. G. Sligar,
and P. G. Wolynes, Science, 254, 1598 (1991).
[2] A. Ansari, J. Berendzen, S.
F. Bowne, H. Frauenfelder, I. E. T. Iben, T. B. Sauke, E. Shyamsunder,
and R. D. Young, Proceedings of the National Academy of Science, 82,
5000 (1985).
[3] K. Fritsch, J. Friedrich, F.
Parak, and J. L. Skinner, Proceedings of the National Academy of Science,
93,
15141 (1996).
[4] C. Hofmann, T. J. Aartsma,
H. Michel, and J. Köhler, Proceedings of the National Academy of Science,
100,
15534 (2003).
[5] A. Zumbusch, L. Fleury, R.
Brown, J. Bernard, and M. Orrit, Physical Review Letters, 70, 3584
(1993).
[6] A. Kiraz, M. Ehrl, Ch. Bräuchle,
A. Zumbusch, Journal of Chemical Physics, 118, 10821 (2003).
[7] E. Hofmann, P. Wrench, F. P.
Sharples, R. G. Hiller, W. Welte, and K. Diedrichs, Science, 272,
1788 (1996).
[8] T. Polívka and V. Sundström,
Chemical Reviews, 104, 2021 (2004) and references therein.
[9] S. M. Mansfield and G. S. Kino,
Applied Physics Letters, 57, 2615 (1990).
[10] S. B. Ippolito, B.B Goldberg,
and S. Unlu, Journal of Applied Physics 97, 053105 (2005).